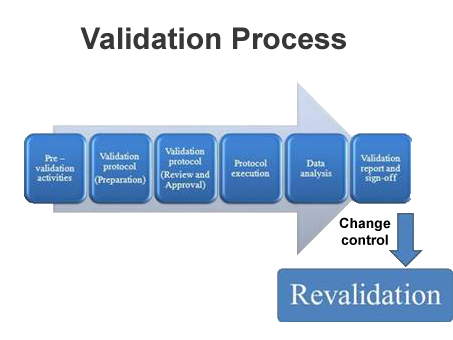
At Contego NutriPharm, we do process validation for Complementary Medicines.
Process validation is defined as the collection and evaluation of data, from the process design stage throughout production, which establishes scientific evidence that a process is capable of consistently delivering quality products.
In situations where no USP methodology is available, we are able to do method validation as well.